The Food and Drug Administration has approved a new device called the Teal Wand, which its creator describes as an "at-home vaginal sample self-collection device for cervical cancer screening." It could be especially useful for women who find pap smears uncomfortable, painful and even traumatic, as well as for those who may not have time to go to a doctor or have disabilities preventing them from traveling to consult one. Users who get the Teal Wand will have to swab their vagina with the sponge tool at its tip. They then have to send the swab in to test it for HPV, or human papillomavirus, which causes most cervical cancers.
Since the user isn't scraping cells from their cervix like what's done with a speculum during pap smears, there are no samples to analyze for abnormality under a microscope. But as The New York Times noted, some authorities are now recommending HPV testing as the primary screening for cervical cancer. Last year, the National Cancer Institute launched a clinical trial network called the Cervical Cancer ‘Last Mile’ Initiative and teamed up with Roche, which provided a similar self-collection solution for participants. The cancer institute explained at the time that cervical cancer is highly preventable with HPV vaccine and regular screening, but half of all diagnosed cases in the US are of individuals who have never been or are infrequently screened. A self-collection device can vastly expand access to cervical cancer screening.
Teal Wand users will be able to dial into the company's telehealth services, with providers who can give them instructions on how to use the device. Users will then have to detach the swab from the wand, put it in a vial, label it and then mail it for testing. Teal Health claims that its solution was proven to be as accurate as in-clinic screening during its clinical study. The company will start shipping out wands to California residents in June before expanding availability across the country. It doesn't have pricing for the device yet, but it said it's working with insurance providers so that the wand could be covered by their plans. The company also intends to work with donors to subsidize costs for people without insurance, as well as to offer flexible payment options.
Login to add comment
Other posts in this group



This week, I chat with Sam Chapman, Engadget’s new security reporter who’s been reviewing VPNs and related products. He dives into what led him to security, the VPNs he likes the most and his thoug

Get ready for your game library to grow, because the Steam Summer Sa
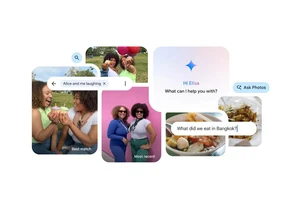
Google has improved its AI-power

Apple has introduced new fee structure